
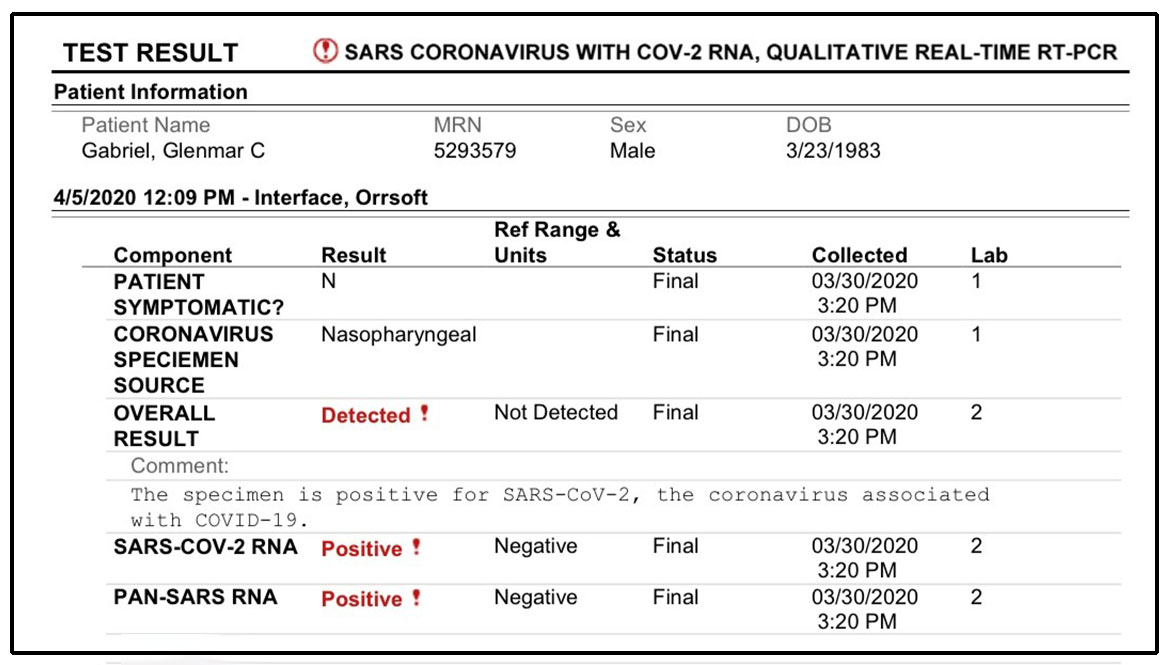
Results are typically visible to the eye, not requiring special equipment. Always seek the advice of your physician or other qualified health providers with any questions you may have regarding a medical condition.
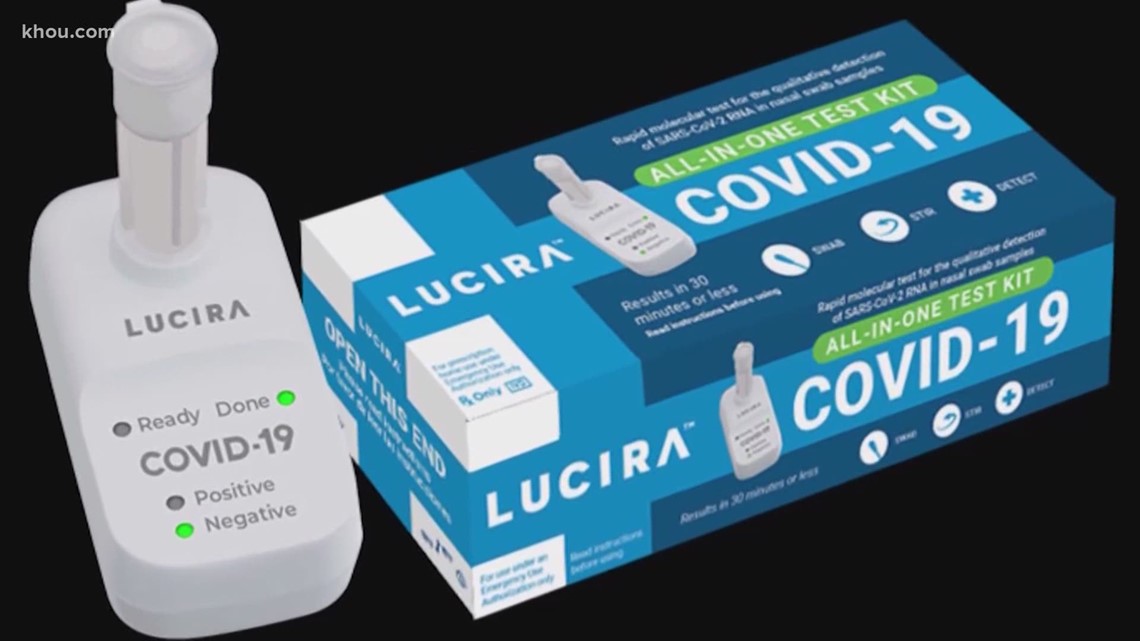
This website is for informational purposes only. This is typically coupled with LAMP, but this is not always necessary. The information on this website is not intended to be a substitute for or be relied upon as medical advice, diagnosis, or treatment. The presence of an active infection, by targeting specific gene sequences of SARS-CoV-2. It is also difficult to quantitfy the results (level of viral infection). It has a very low limit of detection of 125 viruses/mL.ĭesigning the necessary primers can be complex, and debris can interfere with the reaction. It is very rapid and does not always require special equipment. It is also difficult to quantify the results (level of viral infection). It has a very low limit of detection of 125 viruses/mL.ĭesigning the primers needed can be complex, and debris can interfere with the reaction. It is very rapid and does not always require special equipment (can be measured by eye in some cases). It relies on specially designed primers that help create the looped structures needed for amplification. The time needed to complete the test trained personnel and special equipment to analyze the results It typically has a very low limit of detection, around 100 viruses/mL.
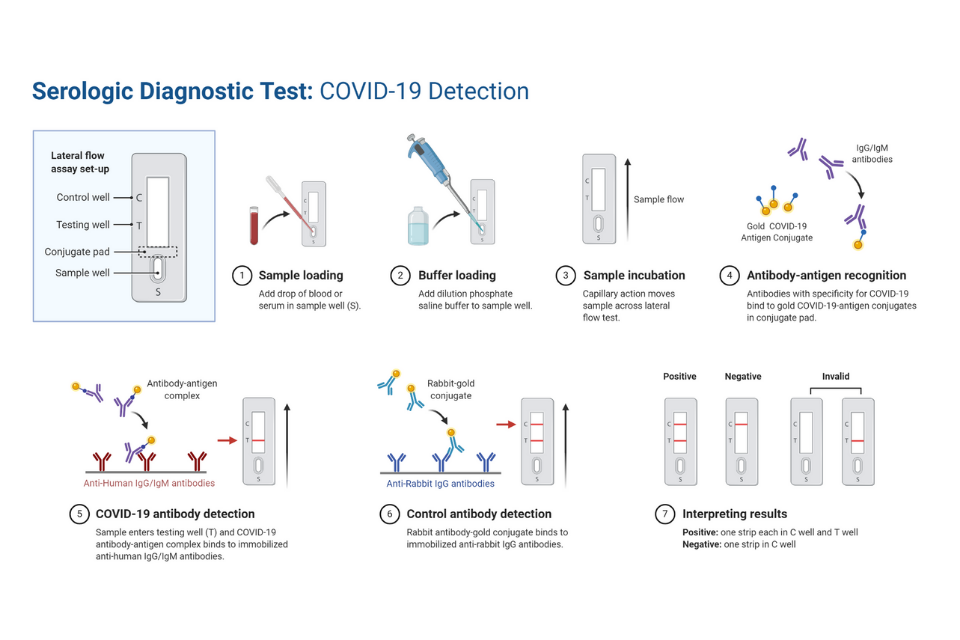
This can be quantitative but is usually qualitative (yes/no). Requires very careful design of synthetic antibodies, deep knowledge of viral proteins produced in various tissue environments, and may yield false negatives if the viral protein production is low. This is typically coupled with lateral flow assays to display results that can be read by eye. Acutis Diagnostics’ Post Acutis Diagnostics 2,773 followers. Earlier this year, Acutis a deal to incorporate Sophia Genetics. The test may only be performed by Hicksville, New York-based Acutis, according to the FDA. This was not only likely but expected given the ability of. Paxlovid got its emergency use authorization in December 2021, for ages 12+ with a high risk for severe COVID. The SARS-CoV-2 Acutis Multiplex Assay is designed for the qualitative detection of nucleic acid from SARS-CoV-2 in nasopharyngeal swab specimens from individuals suspected of having COVID-19. The presence of an active infection, by detecting specific viral proteins present in a patient sample. High seroprevalence was shown when pets from COVID-19 positive households were tested 11. What types of diagnostic tests are on the market? What are the differences between them?įive common types of antigen and molecular tests are currently available: Type of Test


 0 kommentar(er)
0 kommentar(er)
